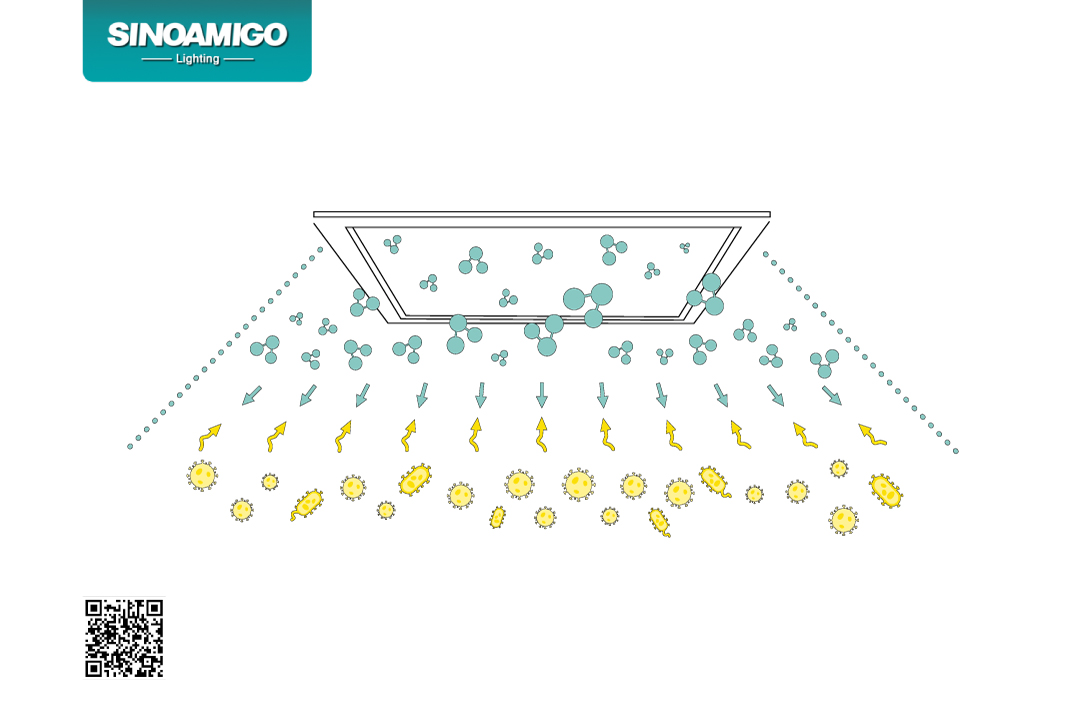
Definition of photocatalysts:
Photocatalysts are a general term for semiconductor materials with photocatalytic functions. It is mainly represented by nano-scale titanium dioxide, which can produce strong oxidizing substances under light irradiation.
Photocatalytic reaction:
When photocatalyst materials are exposed to light, a photocatalytic reaction similar to photosynthesis will occur. During the reaction, nanomaterials will absorb light energy, thereby stimulating oxygen molecules in the air to produce free hydroxyl radicals and reactive oxygen.
Role of oxidants:
The oxidants produced by photocatalysts have extremely strong oxidizing ability. They can decompose various organic compounds and some inorganic substances, while destroying bacterial cell membranes and solidifying viral proteins.
Reaction mechanism:
The photocatalytic oxidation reaction of photocatalyst is based on the energy band theory of N-type semiconductors. When light is irradiated on the photocatalyst material, the electrons in the valence band will be excited to jump to the conduction band, and corresponding electron holes will be generated in the valence band. These electron holes have a strong electron-absorbing ability and can oxidize and decompose the substances adsorbed on the surface of the photocatalyst.
Application field:
Photocatalysts are often used in home air purification, medical and health fields, etc., and can effectively degrade toxic and harmful gases in the air, such as formaldehyde, and kill a variety of bacteria.
In summary, the principle of photocatalyst materials mainly depends on their photocatalytic effect, which decomposes organic compounds, some inorganic substances, and kills bacteria by absorbing light energy to produce strong oxidizing substances. This principle makes photocatalysts show strong application potential in many fields.
Post time: Jun-28-2024